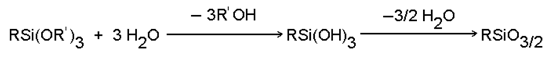| |
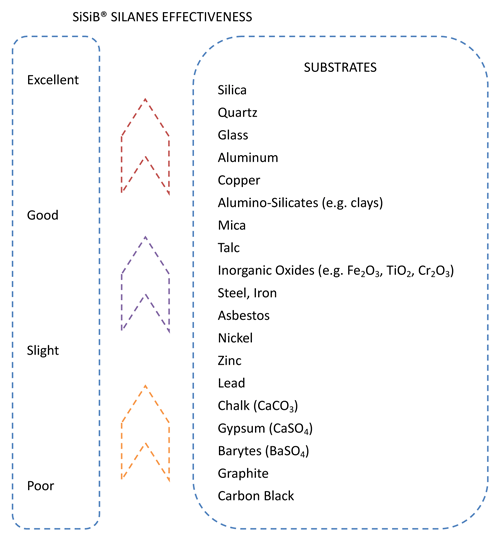
| Much more effect |
Silica, alumina, glass, quartz, porcelain clay |
| Much effect |
Mica, talc, clay, water and alumina, grammiteiron dust, potassium titanic acid |
| Ratherish effect |
Asbestos, ferric oxide, zinc oxide, carborundum, silicon nitride |
| Not very effect |
calcium carbonate, carbon, barium sulphate,boron |
 |
| |
There are three general methods for treating the surfaces of inorganic filler materials before they are added to the organic resins.
 |
Wet Method |
| |
By mixing a slurry of the inorganic materials in a dilute solution of the silane coupling agent, a highly uniform and precise surface treatment of the inorganic material can be obtained. |
 |
Dry Method |
| |
A high shear, high speed, mixer is used to disperse the silane coupling agent into the inorganic materials. The silane is generally applied either neat or as a concentrated solution. When compared to the Wet Method, the Dry Method is most often preferred for large-scale production, treating a large amount of filler in a relatively short time and generating relatively little mixed waste; however, it is more difficult to obtain uniform treatment with this method. |
 |
Spray Method |
| |
Spray the silane coupling agent on high temperature filler that was just taken out from furnace. The method may omit dry procedure and make the process simplify, but pay attention to perflation and ignite. |
|